"Viral Vectors-Based Gene Therapy for Non-Human Primates Market Size, Share, and Trends Analysis Report—Industry Overview and Forecast to 2032
According to Data Bridge Market Research firms, the Gene Delivery Systems Market is set to achieve robust growth, supported by emerging economies and digital transformation. Companies operating in the Viral Gene Transfer Market are leveraging advanced technologies to enhance productivity and meet consumer expectations. The demand for customized solutions is rising, further driving expansion in the Genetic Therapy for Primate Models Market. Leading industry players are focusing on research-backed strategies to strengthen their market position. As competition intensifies, businesses in the Adeno-Associated Virus (AAV) Therapy Market are utilizing detailed market research reports to understand shifting trends, consumer behavior, and future opportunities in the Retroviral Vector Therapeutics Market.
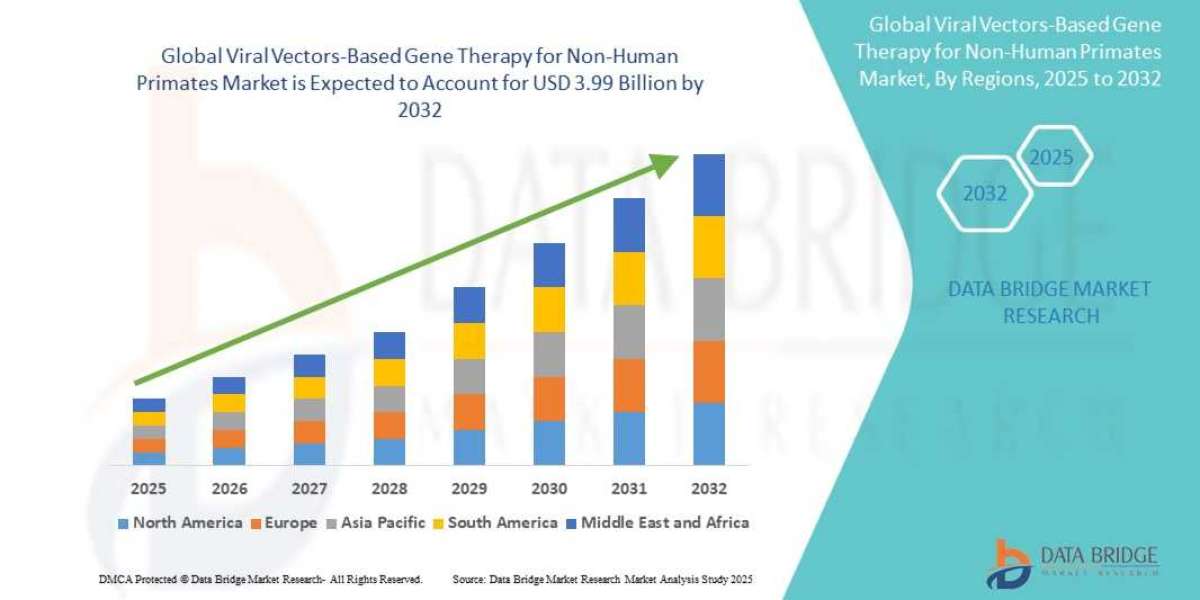
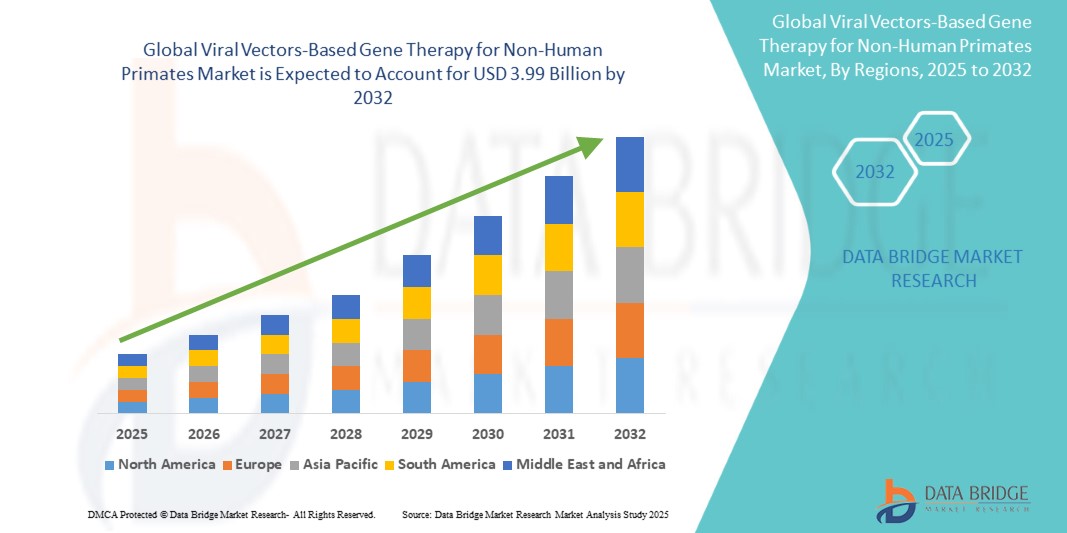
The Viral Vectors-Based Gene Therapy for Non-Human Primates Market is poised for significant growth, with a market outlook highlighting substantial growth potential driven by emerging opportunities in key sectors. This report provides strategic insights, demand dynamics, and revenue projections, offering a comprehensive view of the future landscape, technology disruptions, and adoption trends shaping the industry’s ecosystem evaluation. According to Data Bridge Market Research Global viral vectors-based gene therapy for non-human primates market size was valued at USD 1.26 billion in 2024 and is projected to reach USD 3.99 billion by 2032, with a CAGR of 15.40% during the forecast period of 2025 to 2032.
Leading market research reports highlight the growing use of advanced solutions in the Lentiviral Gene Therapy Market to improve efficiency and sustainability. Businesses are adapting to regulations, integrating technology, and refining their strategies to stay competitive in the Preclinical Gene Therapy Market. The rise of digital transformation has reshaped the Primate Model Genetic Research Market, pushing companies to invest in automation and smarter business models. With demand rising, companies in the Viral-Based DNA Therapy Market are focusing on innovation and customer engagement to stand out. As the industry expands, the Recombinant Viral Vector Market presents endless possibilities for businesses ready to embrace change.
Our comprehensive Viral Vectors-Based Gene Therapy for Non-Human Primates Market report is ready with the latest trends, growth opportunities, and strategic analysis. https://www.databridgemarketresearch.com/reports/global-viral-vectors-based-gene-therapy-for-non-human-primates-market
**Segments**
- **By Vector Type**: Adeno-Associated Virus Vectors, Adenoviral Vectors, Lentiviral Vectors, Retroviral Vectors, Other Viral Vectors
- **By Application**: Gene Therapy, Vaccinology
- **By End User**: Research Institutes, Biopharmaceutical Companies, Contract Research Organizations (CROs)
The global viral vectors-based gene therapy for non-human primates market is segmented based on vector type, application, and end users. This segmentation allows for a more comprehensive understanding of the market dynamics and key areas of growth. Adeno-associated virus vectors, adenoviral vectors, lentiviral vectors, retroviral vectors, and other viral vectors are the main types of vectors used in gene therapy for non-human primates. The application segment includes gene therapy and vaccinology, catering to various therapeutic needs in this market. Additionally, the end-user segment comprises research institutes, biopharmaceutical companies, and contract research organizations (CROs) that actively contribute to the development and commercialization of viral vectors-based gene therapy for non-human primates.
**Market Players**
- Sanofi
- Pfizer Inc.
- Novartis AG
- Gilead Sciences, Inc.
- bluebird bio, Inc.
- Lonza
- Spark Therapeutics, Inc.
- The Jackson Laboratory
- Addgene
- Vigene Biosciences
Several key players are driving the global viral vectors-based gene therapy for non-human primates market. Companies such as Sanofi, Pfizer Inc., Novartis AG, Gilead Sciences, Inc., and bluebird bio, Inc. are at the forefront of innovation in this field. Furthermore, Lonza, Spark Therapeutics, Inc., The Jackson Laboratory, Addgene, and Vigene Biosciences are also significant contributors to the market, providing advanced solutions and technologies for gene therapy applications in non-human primates. These market players are actively engaged in research and development activities, strategic collaborations, and product launches to maintain their competitive edge and expand their market presence.
https://www.databridgemarketresearch.com/reports/global-viral-vectors-based-gene-therapy-for-non-human-primates-market The global viral vectors-based gene therapy for non-human primates market is currently witnessing significant growth and evolving dynamics driven by technological advancements and increasing research in the field of gene therapy and vaccinology. With a focus on delivering innovative solutions for non-human primates, various segments within this market are playing a crucial role in shaping the industry landscape. The use of different vector types such as adeno-associated virus vectors, adenoviral vectors, lentiviral vectors, retroviral vectors, and other viral vectors showcases the diversity in approaches and applications in gene therapy for non-human primates. Each vector type offers distinct advantages and may be tailored to specific therapeutic requirements, highlighting the importance of a diversified portfolio in this market.
In terms of applications, gene therapy and vaccinology are key areas driving the adoption of viral vectors-based gene therapy for non-human primates. Gene therapy holds promise in addressing genetic disorders and diseases in non-human primates through targeted gene delivery, while vaccinology plays a vital role in developing vaccines for infectious diseases and other health conditions. This dual focus on therapeutic innovation and preventive medicine underscores the significance of viral vectors in advancing healthcare solutions for non-human primates.
The end-user segment comprising research institutes, biopharmaceutical companies, and contract research organizations (CROs) reflects a collaborative ecosystem driving research, development, and commercialization efforts in the viral vectors-based gene therapy market for non-human primates. Research institutes contribute valuable insights and expertise, while biopharmaceutical companies bring industry experience and resources to accelerate product development and market penetration. Contract research organizations play a pivotal role in facilitating preclinical and clinical research activities, offering specialized services and infrastructure to support the advancement of viral vectors-based gene therapy for non-human primates.
Leading market players such as Sanofi, Pfizer Inc., Novartis AG, Gilead Sciences, Inc., bluebird bio, Inc., Lonza, Spark Therapeutics, Inc., The Jackson Laboratory, Addgene, and Vigene Biosciences are driving innovation and growth in the global viral vectors-based gene therapy for non-human primates market. These companies are investing in research and development, forging strategic partnerships, and leveraging their technological capabilities to bring cutting-edge solutions to the market. As the market continues to expand and evolve, collaborations among key stakeholders and ongoing investments in advanced technologies will be instrumental in shaping the future trajectory of viral vectors-based gene therapy for non-human primates.**Segments**
Global Viral Vectors-Based Gene Therapy for Non-Human Primates Market Segmentation:
- Type of Viral Vectors: Adenoviral Vectors, Adeno-Associated Viral (AAV) Vectors, Lentiviral Vectors, Retroviral Vectors, Others
- Delivery Method: In Vivo Gene Therapy, Ex Vivo Gene Therapy
- Source: Recombinant Viral Vectors, Native Viral Vectors
- Application: Oncology, Neurological Disorders, Genetic Disorders, Cardiovascular Diseases, Infectious Diseases, Others
- End User: Pharmaceutical Companies, Biotechnology Companies, Research Institutions, Contract Research Organizations (CROs) – Industry Trends and Forecast to 2032
**Market Players**
- Spark Therapeutics, Inc. (U.S.)
- Adenovirus Vectors, Inc. (U.S.)
- Oxford Biomedica plc (UK)
- Viral Vectors, Inc. (U.S.)
- Bluebird Bio, Inc. (U.S.)
- Regenxbio Inc. (U.S.)
- Genocea Biosciences, Inc. (U.S.)
- CureVac AG (Germany)
- Boehringer Ingelheim (Germany)
- Thermo Fisher Scientific Inc. (U.S.)
- Lonza Group AG (Switzerland)
- Charles River Laboratories International, Inc. (U.S.)
- Merck KGaA (Germany)
- Catalent, Inc. (U.S.)
- Sartorius AG (Germany) among others
The global viral vectors-based gene therapy for non-human primates market is witnessing significant growth propelled by technological advancements and increasing research in gene therapy and vaccinology. The segmentations based on viral vector types, delivery methods, sources, applications, and end-users provide a comprehensive understanding of the market landscape. Various viral vector types like Adenoviral Vectors, AAV Vectors, Lentiviral Vectors, and Retroviral Vectors serve different therapeutic needs in oncology, neurological disorders, genetic disorders, cardiovascular diseases, and infectious diseases for non-human primates. The diversity in applications and end-user segments involving pharmaceutical companies, biotechnology firms, research institutions, and CROs demonstrate a collaborative ecosystem that drives innovation and commercialization efforts in this space.
Key market players like Spark Therapeutics, Inc., Bluebird Bio, Inc., Lonza Group AG, among others, play a pivotal role in driving innovation and growth in the global viral vectors-based gene therapy market. Strategic collaborations, research investments, and technological advancements by these companies are shaping the future trajectory of viral vectors-based gene therapy. As the industry continues to evolve, the emphasis on advanced technologies and partnerships will be critical in advancing healthcare solutions for non-human primates.
The market is highly fragmented, with a mix of global and regional players competing for market share. To Learn More About the Global Trends Impacting the Future of Top 10 Companies in Viral Vectors-Based Gene Therapy for Non-Human Primates Market : https://www.databridgemarketresearch.com/reports/global-viral-vectors-based-gene-therapy-for-non-human-primates-market/companies
Key Questions Answered by the Global Viral Vectors-Based Gene Therapy for Non-Human Primates Market Report:
- How will the increasing adoption of Viral Vectors-Based Gene Therapy for Non-Human Primates Market in high-performance computing impact the overall market growth?
- How much is the global Viral Vectors-Based Gene Therapy for Non-Human Primates Market worth? What was the market value in 2024?
- Who are the major players operating in the Viral Vectors-Based Gene Therapy for Non-Human Primates Market? Which companies are the front runners?
- Which recent industry trends can be implemented to generate additional revenue streams?
- How will AI, IoT, and 5G advancements influence the Viral Vectors-Based Gene Therapy for Non-Human Primates Market in the next five years?
- What are the key drivers fueling the growth of the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
- What are the major challenges and barriers faced by the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
- How is technological innovation shaping the future of Viral Vectors-Based Gene Therapy for Non-Human Primates Market products?
- What is the impact of government regulations and policies on the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
- How do supply chain disruptions affect the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
- What are the regional differences in demand for Viral Vectors-Based Gene Therapy for Non-Human Primates Market products?
- How do revenue streams vary across different sectors of the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
- What role does technology play in enhancing growth and efficiency in the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
Browse More Reports:
https://www.databridgemarketresearch.com/reports/global-degaussing-system-market
https://www.databridgemarketresearch.com/reports/global-waterborne-coating-additives-market
https://www.databridgemarketresearch.com/reports/global-sulfate-potash-market
https://www.databridgemarketresearch.com/reports/global-api-testing-market
https://www.databridgemarketresearch.com/reports/global-ethylene-vinyl-alcohol-copolymer-evoh-packaging-films-market
Data Bridge Market Research:
☎ Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 982
✉ Email: corporatesales@databridgemarketresearch.com
Tag
Viral Vectors-Based Gene Therapy for Non-Human Primates Market Size, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Share, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Trend, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Analysis, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Report, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Growth, Latest Developments in Viral Vectors-Based Gene Therapy for Non-Human Primates Market, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Industry Analysis, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Key Players, Viral Vectors-Based Gene Therapy for Non-Human Primates Market Demand Analysis"